November 2020 update. Phase 2a study begun in South Africa. 11/25/2020 https://clinicaltrials.gov/show/NCT04044001
August 2019 update. Planned study: Multiple Ascending Dose (MAD) to Evaluate Safety, Tolerability and Early Bactericidal Activity at TASK Applied Science in Cape Town, South Africa.
August 2018. Phase 1a completed
June 2018 update. Phase 1 trial started 26 June 2018.
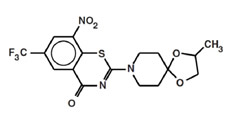
BTZ-043 efficiently inhibits Mtb cell wall synthesis by blocking the decaprenyl- phosphoribose-2′-epimerase (DprE1), necessary for the synthesis of D-Arabinofuranose, a component of arabinogalactan and arabinomannan. Its mechanism of action is highly selective for mycobacterial species. BTZ-043 is active against all tested Mtb strains including clinical isolated from MDR and XDR patients. The in vitro MIC ranges between ~0.1 - 80 ng/ml for fast growers, and from 1 - 30 ng/ml for members of the M. tuberculosis complex. In vivo BTZ-043 shows superior activity to INH in mouse models, most prominent after 2 months and thereafter. Synergistic effects with rifampicin and bedaquiline were detected. In preclinical toxicology (GLP) studies, BTZ-043 showed a low toxicologic potential, it was well tolerated up to 170 mg/kg (NOAEL) in rats over 28 days and it showed a NOAEL of 360mg/kg in minipigs. BTZ-043 showed low interaction with the CYP450 enzymes. The safety panel (neurotoxicity, cardiotoxicity and respiratory toxicity) was conducted under GLP standards and within the NOAELs determined in the toxicology studies; no negative effects were observed. Phototoxicity, genotoxicity and mutagenicity studies were negative. A 6 months chronic toxicity study in rats is ongoing. In vitro metabolism studies implicate an acceptable stability in the human organism with no human-specific metabolite. Metabolism elucidation was performed in a hepatocyte assay as well as in a bile duct fistula study. Metabolism and excretion pathways have been determined. A 8kg GMP batch of drug substance is available, high purity of the substance can be achieved easily. A preliminary formulation for the Phase I clinical trial was developed. CTA for the first-in-human single ascending dose study was submitted to the authorities 1st of March 2018.