Phase 1a began January 16, 2019. Placebo-Controlled, Randomized, SAD Study to Evaluate the Safety, Tolerability, and PK of TBI-223 in Healthy NCT03758612
Presented at ASM Microbe 2017 Poster 50 Sunday:
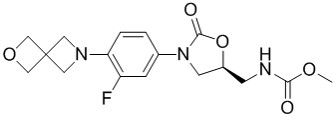
TBI-223, a novel oxazolidinone, has the following properties: ADME: TBI-223 has improved stability in microsomes and hepatocytes from 5 species tested; no inhibition of the 5 major human CYPs (20% at 30 μM); and no CYP induction in human hepatocytes or activation of PXR/CAR/AhR. Pharmacokinetics: TBI-223 has high oral bioavailability and a reasonable volume of distribution in mice and a relatively short half-life of 3 hr; a high bioavailability in rats with a reasonable volume of distribution and a longer half-life of 8 hr; a high oral bioavailability in dogs and moderate clearance at 6.6 mL/min/kg; and a projected human efficacious dose of 800 mg QD. Pharmacology: TBI-223 has activity against drug-sensitive and drug-resistant Mtb strains including clinical strains from all global lineages; activity against Gram-positives and non-tuberculous mycobacteria; efficacy in mouse TB infections; and additive activity in combination with Bedaquiline and Pretomanid, with a potential to replace LZD in the NiX regimen and deliver a universal treatment shortening regimen. Safety: TBI-223 has significantly reduced inhibition of mammalian MPS with an IC50 of >74 μM, versus an IC50 of 8 μM for LZD; no hematological changes or bone marrow toxicity observed in a 28-day rat toxicity study with an AUC of 1685 µg·hr/mL which is 10 fold higher than the efficacious exposure mice. In contrast the safety margin for bone marrow toxicity of LZD was less than 1. No bone marrow toxicity was observed in a 14-day dog toxicity study of TBI-223 at the highest dose of 150 mg/Kg QD tested (AUC of 789 µg·hr/mL).
In a 14-day non-GLP rat study the NOAEL for TBI-223 was 200 mg/kg/day for males, and 75 mg/kg/day for females compared to a NOAEL of 20 mg/kg/day for LZD. At the NOAEL the AUC of TBI-223 for females and males was 3X and 6X MED, respectively. In the 14-day dog non-GLP toxicity study the NOAEL for TBI-223 was 150 mg/Kg/day. TBI-223 is safer than LZD for TB therapy within a treatment shortening regimen, and is currently in preclinical development.