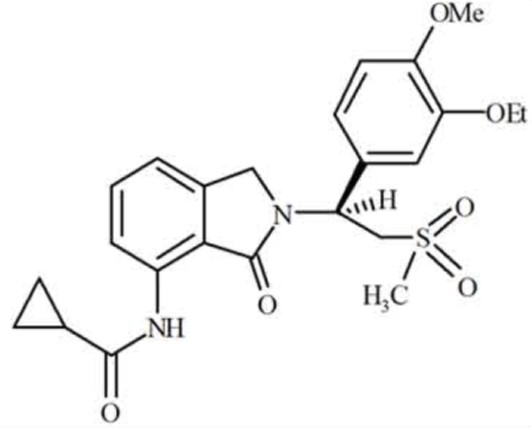
CC-11050 is a novel anti-inflammatory compound with potential to treat a variety of chronic inflammatory conditions and cytokine storms associated with infectious diseases. CC-11050 is a selective phosphodiesterase-4 inhibitor (PDE4) that is active in several in vivo models of inflammatory disease, inhibiting systemic TNF- production, colitis symptoms of the colon, psoriasiform features in the skin, arthritogenic swelling in the joints, neutrophilia and eosinophilia in the lung, and reducing choroidal neovascularization.
CC-11050 plus isoniazid therapy significantly reduced bacillary load and pathology in a rabbit model pulmonary tuberculosis. Subbian et al. 2016. Adjunctive phosphodiesterase-4 inhibitor therapy improves antibiotic resonse to pulmonary tuberculsis in a rabbit model. EBioMedicine 4:104
*Update 02 September 2022*
- Medicines Development for Global Health announced the licensing-in of CC-11050 from Amgen on 23 December 2020. The compound recently completed Phase 2a clinical trials for tuberculosis (NCT02968927) and stage 1 of a two stage trial for leprosy type 2 reaction (also known as erythema Nodosum Leprosum, an inflammatory complication of leprosy) and is set to start additional Phase 2 trials shortly.
- Medicines Development for Global Health is a non-profit, independent biopharmaceutical company dedicated to the development and delivery of new and improved medicines for diseases that disproportionately affect people in low- and middle-income countries.
- Under the terms of the agreement with Amgen, Medicines Development for Global Health has assumed full responsibility for the further development and commercialization of CC-11050 and has exclusive, worldwide rights for its potential applications in all neglected tropical diseases.
- Amgen will continue to support the two Phase 2 clinical trials in leprosy (conducted by The Leprosy Mission Nepal) and TB (conducted by the Aurum Institute NPC) by providing CC-11050 for both trials and funding the leprosy type 2 reaction study.