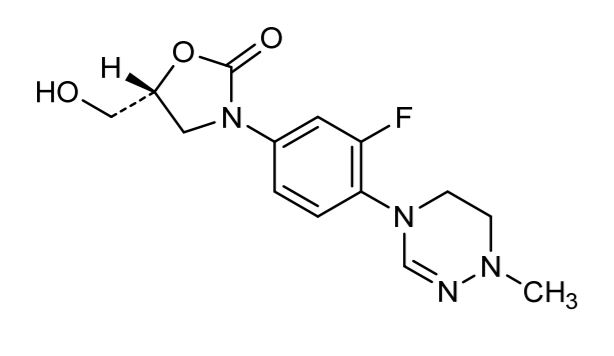
A novel oxazolidinone with cyclic amidrazone synthesized by LegoChem BioSciences Inc. (Daejeon, Republic of Korea).
February 2022. Early Bactericidal Activity of Delpazolid (LCB01-0371) in Patients with Pulmonary Tuberculosis. Kim JS, Kim YH, Lee SH, Kim YH, Kim JW, Kang JY, Kim SK, Kim SJ, Kang YS, Kim TH, Mok J, Byun MK, Park HJ, Joh JS, Park YB, Lim HS, Choi H, Lee SH, Kim H, Yang J, Kim H, Shen X, Alsultan A, Cho I, Geiter L, Shim TS. Antimicrob Agents Chemother. 2022 Feb 15;66(2):e0168421.
The average daily decline in log-CFU was 0.044 ± 0.016, 0.053 ± 0.017, 0.043 ± 0.016, and 0.019 ± 0.017, for the delpazolid 800 mg QD, 400 mg BID, 800 mg BID, and the 1,200 mg QD groups, respectively. The average daily decline in log-CFU was 0.192 ± 0.028 for the HRZE group and 0.154 ± 0.023 for the linezolid 600 mg BID group. Three serious adverse events (SAE) were reported, one each in the delpazolid 400 mg BID group (death due to worsening of TB at day 2), the HRZE group (hospitalization due to pleural effusion) and the linezolid group (hyperkalemia); none of the SAEs were assessed as related to study drugs. NCT02836483
October 2021. Phase 2b study began in South Africa and Tanzania in collaboration with PanACEA, Ludwig-Maximilians - University of Munich, Radboud University Medical Center, and the University of California, San Francisco. DECODE https://clinicaltrials.gov/show/NCT04550832
| Estimated Primary Completion Date : | May 30, 2022 |
| Estimated Study Completion Date : | January 30, 2023 |
July 2018. Comparison of in vitro activity and MIC distributions between the novel oxazolidinone delpazolid and linezolid against multidrug-resistant and extensively drug-resistant Mycobacterium tuberculosis in China. Zong Z, Jing W, Shi J, Wen S, Zhang T, Huo F, Shang Y, Liang Q, Huang H, Pang Y. Antimicrob Agents Chemother. 2018 May 29. pii: AAC.00165-18. doi: 10.1128/AAC.00165-18.
January 2018. Safety, tolerability and pharmacokinetics of 21 day multiple oral administration of a new oxazolidinone antibiotic, LCB01-0371, in healthy male subjects. Choi Y, Lee SW, Kim A, Jang K, Nam H, Cho YL, Yu KS, Jang IJ, Chung JY. J Antimicrob Chemother. 2018 Jan 1;73(1):183-190. doi: 10.1093/jac/dkx367.
June 2017. Enrolling Phase 2 tuberculosis clinical study in Korea. https://clinicaltrials.gov/show/NCT02836483
December 2010. In vitro and in vivo activities of LCB01-0371, a new oxazolidinone. Jeong JW, Jung SJ, Lee HH, Kim YZ, Park TK, Cho YL, Chae SE, Baek SY, Woo SH, Lee HS, Kwak JH. Antimicrob Agents Chemother. 2010 Dec;54(12):5359-62. doi: 10.1128/AAC.00723-10. Epub 2010 Sep 20. PMID: 20855730
Abstract: LCB01-0371 is a new oxazolidinone with cyclic amidrazone. In vitro activity of LCB01-0371 against 624 clinical isolates was evaluated and compared with those of linezolid, vancomycin, and other antibiotics. LCB01-0371 showed good activity against Gram-positive pathogens. In vivo activity of LCB01-0371 against systemic infections in mice was also evaluated. LCB01-0371 was more active than linezolid against these systemic infections. LCB01-0371 showed bacteriostatic activity against Staphylococcus aureus.