https://doi.org/10.1016/j.tube.2021.102104
Parvinder Kaur, Vijay Potluri, Vijay Kamal Ahuja, C.N. Naveenkumar, Ramya Vadageri Krishnamurthy, Shruthi Thimmalapura Gangadharaiah, Prasad Shivarudraiah, Sumesh Eswaran, Christy Rosaline Nirmal, Balasubramanian Mahizhaveni, Azger Dusthackeer, Rajesh Mondal, Sarah M. Batt, Emily J. Richardson, Nicholas J. Loman, Gurdyal Singh Besra, Radha Krishan Shandil, Shridhar Narayanan.
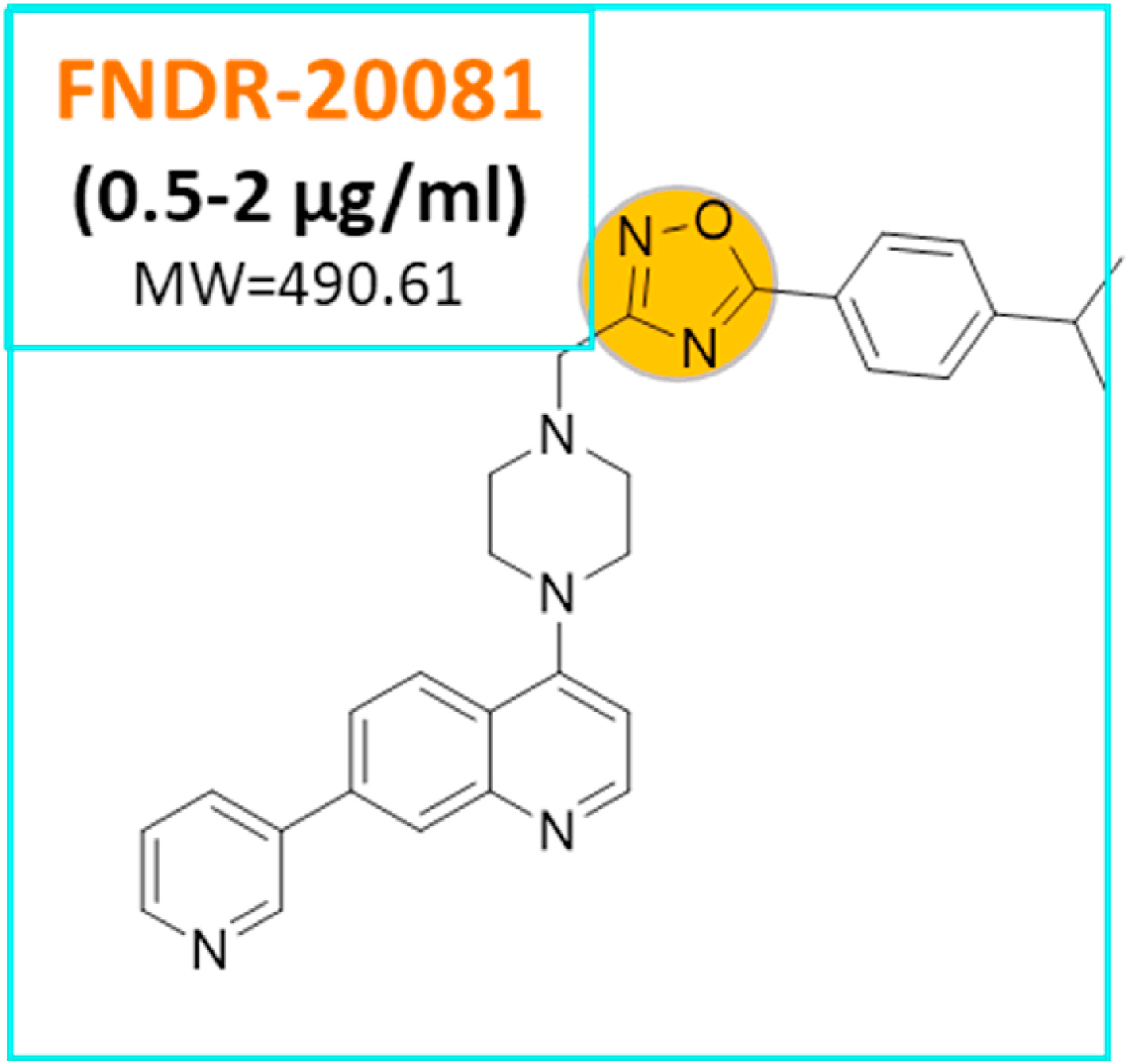
A multi-targeting pre-clinical candidate against drug-resistant tuberculosis.
Tuberculosis, Volume 129, 2021, 102104, ISSN 1472-9792. PMID: 34214859
Abstract
"FNDR-20081 [4-{4-[5-(4-Isopropyl-phenyl)- [1,2,4]oxadiazol-3-ylmethyl]-piperazin-1-yl}-7-pyridin-3-yl-quinoline] is a novel, first in class anti-tubercular pre-clinical candidate against sensitive and drug-resistant Mycobacterium tuberculosis (Mtb). In-vitro combination studies of FNDR-20081 with first- and second-line drugs exhibited no antagonism, suggesting its compatibility for developing new combination-regimens. FNDR-20081, which is non-toxic with no CYP3A4 liability, demonstrated exposure-dependent killing of replicating-Mtb, as well as the non-replicating-Mtb, and efficacy in a mouse model of infection. Whole genome sequencing (WGS) of FNDR-20081 resistant mutants revealed the identification of pleotropic targets: marR (Rv0678), a regulator of MmpL5, a transporter/efflux pump mechanism for drug resistance; and Rv3683, a putative metalloprotease potentially involved in peptidoglycan biosynthesis. In summary, FNDR-20081 is a promising first in class compound with the potential to form a new combination regimen for MDR-TB treatment."