MBio. 2018 Dec 18;9(6). pii: e02101-17. doi: 10.1128/mBio.02101-17. Synergistic Lethality of a Binary Inhibitor of Mycobacterium tuberculosis KasA.
Kumar P#1, Capodagli GC#2, Awasthi D#3, Shrestha R1, Maharaja K2, Sukheja P1, Li SG3, Inoyama D3, Zimmerman M4, Ho Liang HP4, Sarathy J4, Mina M4, Rasic G4, Russo R1, Perryman AL3, Richmann T1, Gupta A1, Singleton E1, Verma S1, Husain S2,5, Soteropoulos P2,5, Wang Z6, Morris R6, Porter G7, Agnihotri G7, Salgame P1, Ekins S8, Rhee KY6, Connell N1, Dartois V4, Neiditch MB#9, Freundlich JS#10,3, Alland D#10.
Author information:
1. Division of Infectious Disease, Department of Medicine, and the Ruy V. Lourenco Center for the Study of Emerging and Reemerging Pathogens, New Jersey Medical School, Rutgers, The State University of New Jersey, Newark, New Jersey, USA; 2. Department of Microbiology, Biochemistry and Molecular Genetics, New Jersey Medical School, Rutgers, The State University of New Jersey, Newark, USA; 3. Department of Pharmacology, Physiology & Neuroscience, New Jersey Medical School, Rutgers, The State University of New Jersey, Newark, New Jersey, USA; 4. Public Health Research Institute, New Jersey Medical School, Rutgers, The State University of New Jersey, Newark, New Jersey, USA;5. Genomics Center, New Jersey Medical School, Rutgers, The State University of New Jersey, Newark, New Jersey, USA.
6. Division of Infectious Diseases, Department of Medicine, Weill Cornell Medical College, New York, New York, USA; 7. WuXi AppTec, Plainsboro, New Jersey, USA.
8. Collaborations Pharmaceuticals, Inc., Raleigh, North Carolina, USA; 9. Department of Microbiology, Biochemistry and Molecular Genetics, New Jersey Medical School, Rutgers, The State University of New Jersey, Newark, USA matthew.neiditch@njms.rutgers.edu freundjs@rutgers.edu allandda@njms.rutgers.edu; 10. Division of Infectious Disease, Department of Medicine, and the Ruy V. Lourenco Center for the Study of Emerging and Reemerging Pathogens, New Jersey Medical School, Rutgers, The State University of New Jersey, Newark, New Jersey, USA. matthew.neiditch@njms.rutgers.edu freundjs@rutgers.edu allandda@njms.rutgers.edu.
#. Contributed equally
Abstract
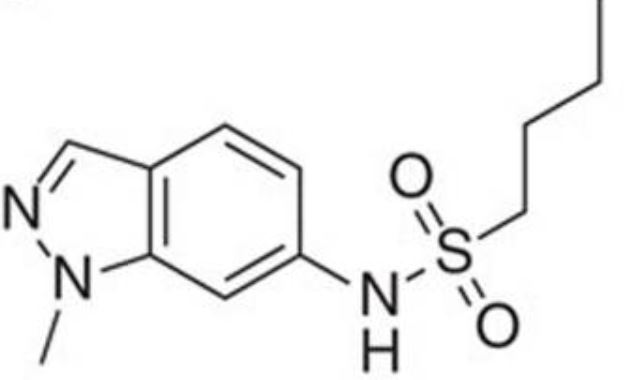
We report GSK3011724A (DG167) as a binary inhibitor of β-ketoacyl-ACP synthase (KasA) in Mycobacterium tuberculosis Genetic and biochemical studies established KasA as the primary target. The X-ray crystal structure of the KasA-DG167 complex refined to 2.0-Å resolution revealed two interacting DG167 molecules occupying nonidentical sites in the substrate-binding channel of KasA. The binding affinities of KasA to DG167 and its analog, 5g, which binds only once in the substrate-binding channel, were determined, along with the KasA-5g X-ray crystal structure. DG167 strongly augmented the in vitro activity of isoniazid (INH), leading to synergistic lethality, and also synergized in an acute mouse model of M. tuberculosis infection. Synergistic lethality correlated with a unique transcriptional signature, including upregulation of oxidoreductases and downregulation of molecular chaperones. The lead structure-activity relationships (SAR), pharmacokinetic profile, and detailed interactions with the KasA protein that we describe may be applied to evolve a next-generation therapeutic strategy for tuberculosis (TB).IMPORTANCE Cell wall biosynthesis inhibitors have proven highly effective for treating tuberculosis (TB). We discovered and validated members of the indazole sulfonamide class of small molecules as inhibitors of Mycobacterium tuberculosis KasA-a key component for biosynthesis of the mycolic acid layer of the bacterium's cell wall and the same pathway as that inhibited by the first-line antitubercular drug isoniazid (INH). One lead compound, DG167, demonstrated synergistic lethality in combination with INH and a transcriptional pattern consistent with bactericidality and loss of persisters. Our results also detail a novel dual-binding mechanism for this compound as well as substantial structure-activity relationships (SAR) that may help in lead optimization activities. Together, these results suggest that KasA inhibition, specifically, that shown by the DG167 series, may be developed into a potent therapy that can synergize with existing antituberculars.
Nat Commun. 2016 Sep 1;7:12581. doi: 10.1038/ncomms12581. Identification of KasA as the cellular target of an anti-tubercular scaffold.
1 Institute of Microbiology and Infection, School of Biosciences, University of Birmingham, Edgbaston, Birmingham B15 2TT, UK.; 2 GlaxoSmithKline, Gunnels Wood Road, Stevenage SG1 2NY, UK.; 3 Cellzome-a GSK Company, Meyerhofstrasse 1, 69117 Heidelberg, Germany; 4 Diseases of the Developing World, GlaxoSmithKline, Severo Ochoa 2, Tres Cantos, 28760 Madrid, Spain; 5 GlaxoSmithKline, 709 Swedeland Road, PO Box 1539, King of Prussia, Pennsylvania 19406-0939, USA; 6 Centre National de la Recherche Scientifique FRE 3689, Centre d'études d'agents Pathogènes et Biotechnologies pour la Santé, Université de Montpellier, 1919 route de Mende, 34293 Montpellier, France; 7 INSERM, CPBS, 34293 Montpellier, France.
Abstract
Phenotypic screens for bactericidal compounds are starting to yield promising hits against tuberculosis. In this regard, whole-genome sequencing of spontaneous resistant mutants generated against an indazole sulfonamide (GSK3011724A) identifies several specific single-nucleotide polymorphisms in the essential Mycobacterium tuberculosis β-ketoacyl synthase (kas) A gene. Here, this genomic-based target assignment is confirmed by biochemical assays, chemical proteomics and structural resolution of a KasA-GSK3011724A complex by X-ray crystallography. Finally, GSK3011724A-resistant mutants increase the in vitro minimum inhibitory concentration and the in vivo 99% effective dose in mice, establishing in vitro and in vivo target engagement. Surprisingly, the lack of target engagement of the related β-ketoacyl synthases (FabH and KasB) suggests a different mode of inhibition when compared with other Kas inhibitors of fatty acid biosynthesis in bacteria. These results clearly identify KasA as the biological target of GSK3011724A and validate this enzyme for further drug discovery efforts against tuberculosis.