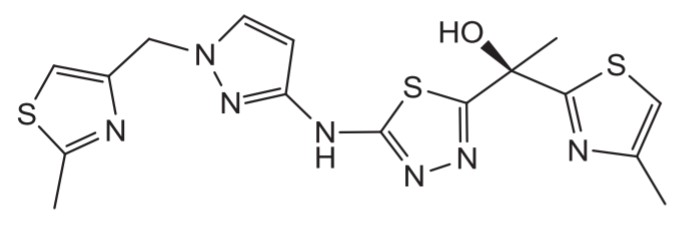
Isoniazid, a first-line drug currently used to treat TB, is activated within the mycobacterial cell by the KatG catalase. The activated molecule is thought to suppress mycolic acid biosynthesis through the inhibition of InhA, an enzyme involved in fatty acid biosynthesis. This makes InhA one of the best validated targets for the treatment of TB. Most isoniazid resistance is mediated through mutations in KatG leading to the inability to activate the drug. The goal is to identify compounds that are able to attain the same clinical efficacy as isoniazid and avoid much of the current resistance to isoniazid by bypassing the requirement for KatG activation and directly inhibiting InhA. GSK screened its corporate compound library to identified hits compounds that were active on the InhA enzyme and progressed them into leads that have anti-mycobacterial activity under the TB Alliance partnership. The thiadiazoles series was identified and become the subject of an optimization effort to generate an orally-administered, safe and efficacious new drug. Since 2011, this optimization effort is done within ORCHID. ORCHID is a TB drug discovery consortium, funded by the EU FP7 program, composed of 12 partners across three continents and leaded by GSK as coordinator. The optimization of this potent and selective class of bactericidal direct inhibitors of Mycobacterium tuberculosis InhA provided compounds orally efficacious in Mtb infections in mouse models. Currently, a thiadiazole is being used to deepen the knowledge of direct InhA inhibitors in combination.
This research has been possible thanks to the collaboration and funding from the Global Alliance for TB Drug Development, and from the European Union's 7th framework program (FP7- 2007-2013) under the Orchid grant agreement No. 261378.
Compound
GSK-693
Description
Related Links