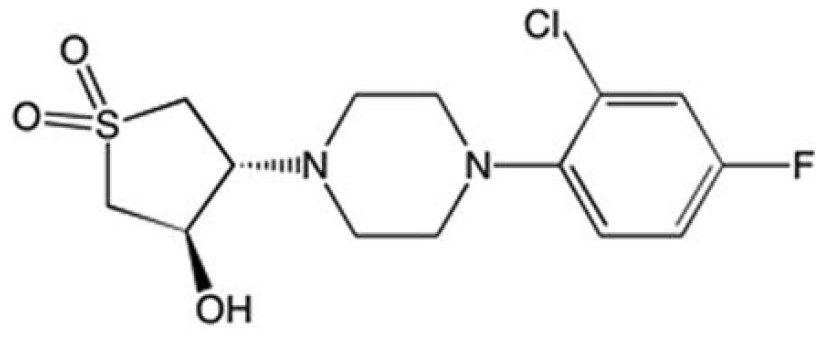
From the publication abstract: Drug discovery eforts against the pathogen Mycobacterium tuberculosis (Mtb) have been advanced through phenotypic screens of extensive compound libraries. Such a screen revealed sulfolane 1 and indoline-5-sulfonamides 2 and 3 as potent inhibitors of mycobacterial growth. Optimization in the sulfolane series led to compound 4, which has proven activity in an in vivo murine model of Mtb infection. The target and mode of inhibition of these compounds was identified based on whole genome sequencing of spontaneous resistant mutants, which identifed mutations locating to the essential α- and β-subunits of tryptophan synthase. Over-expression studies confrmed tryptophan synthase as the biological target. Biochemical techniques probed the mechanism of inhibition, revealing the mutant enzyme complex incurs a ftness cost but does not prevent inhibitor binding. Mapping of the resistance conferring mutations onto a low-resolution crystal structure of Mtb tryptophan synthase showed they locate to the interface between the α- and β-subunits.
Compound
Sulfolane
GlaxoSmithKline
Description
Related Links
Developer Associations