Standard multiple ascending dose Phase I study evaluating PK and safety. 14 day dose-escalation complete; 28 day study in healthy volunteers also completed May 2010.
PNU-100480 is being developed for the treatment of both drug resistant and sensitive tuberculosis. This is an efficacy and safety study to characterize the effect of PNU-100480 when given for 14 days to treatment-naive patients with drug-sensitive pulmonary tuberculosis.
Compound
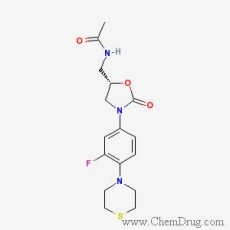
Sutezolid
Sequella, Inc, TB Alliance
Description
Related Links
- 2010 J Infect Dis Pharmacokinetics and whole-blood bactericidal activity against Mycobacterium tuberculosis of single doses of PNU-100480 in healthy volunteers
- 2011 Antimicrob Agents Chemother Biomarker-assisted dose selection for safety and efficacy in early development of PNU-100480 for tuberculosis
- 2011 WGND Annual Meeting--Pfizer presentation
- 2012 WGND Annual Meeting--Pfizer (pre-recorded)presentation
- 2012 WGND Annual Meeting--Pfizer presentation
Regimen Associations
Developer Associations