TB Alliance announced discontinuation March 11, 2016.
"The MAD study, designed to test the tolerability and pharmacokinetics of ascending doses of TBA-354 in healthy volunteers, resulted in side effects in the initial cohort. Based on the observed side effects and pharmacokinetic data of TBA-354 generated in this cohort, TB Alliance together with its scientific advisors made the decision to stop the clinical trial and the clinical development program of TBA-354.
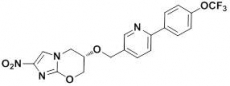
Nitroimidazoles are a known class of chemicals effective against drug-sensitive and drug-resistant M.tb. Pretomanid, a TB drug candidate in late-stage clinical development as a part of the promising PaMZ (Pretomanid + moxifloxacin +pyrazinamide) regimen is a nitroimdazole.
TBA-354 emerged from studies designed to identify a next generation nitroimidazole for TB. TB Alliance conducted the studies in collaboration with the University of Auckland and University of Illinois-Chicago. Once identified, TB Alliance further advanced TBA-354 through pre-clinical development. Having demonstrated advantages over the first-generation compounds, TBA-354 entered clinical testing in 2015.
*From TB Alliance Website http://www.tballiance.org/portfolio/compound/tba-354
Compound
TBA-354
TB Alliance
Description
Related Links
- A review discussing progress of TB drug development.
- Clinical Potential of Nitroimidazole.
- TB Alliance Presentation from 2014 WGND Annual Meeting
- TB Alliance Clinical Development Portfolio
- TB Alliance
- University of Auckland
- Nitroimidazopyran as the drug candidate for the treatment of TB.
- Tasneen et al. 2015. Contribution to regimens in mice. Antimicrob Agents Chemother. Jan;59(1):129-35. doi: 10.1128/AAC.03822-14. Epub 2014 Oct 20. PMID: 25331697
- University of Illinois
Trial Associations
Developer Associations