The repurposing of existing drugs is being pursued as a means by which to accelerate the development of novel regimens for the treatment of drug-susceptible and drug-resistant tuberculosis (TB). In the current study, we assessed the activity of the antipsychotic drug thioridazine (T), in combination with the standard regimen in a well validated murine TB model. Single-dose and steady-state pharmacokinetic studies were performed in BALB/c mice to establish human-equivalent doses of T. To determine the bactericidal activity of T in BALB/c mice with chronic TB, three separate studies were performed, including a dose-ranging study of T monotherapy, and efficacy studies of human-equivalent doses of T with and without isoniazid (H) and rifampin (R). Therapeutic efficacy was assessed by the change in mycobacterial load in the lung. The human-equivalent dose of thioridazine was determined to be 25 mg/kg, which was well tolerated in mice. T was found to accumulate at high concentrations in lung tissue relative to serum levels. We observed modest synergy during co-administration of T with H, and the addition of T reduced the emergence of H-resistant mutants in mouse lungs. In conclusion, this study further illustrates the opportunity to reevaluate the contribution of T to the sterilizing activity of combination regimens to prevent the emergence of drug-resistant M. tuberculosis. Further, we sought to investigate the sterilizing activity of human-equivalent doses of T when given in combination with the 'Denver regimen' against acute murine tuberculosis. We found a trend towards a positive impact of T on the bacterial clearance and lowering relapse rates of the combined standard TB chemotherapy.
Compound
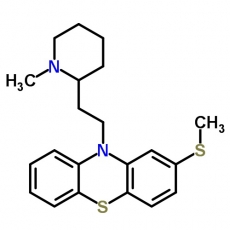
Thioridazine
Description
Related Links
Related Links
- Antimicrob Agents Chemother, 2014 Reduced emergence of isoniazid resistance with concurrent use of thioridazine against acute murine tuberculosis.
- 2010. A Mycobacterium tuberculosis Sigma Factor Network Responds to Cell-Envelope Damage by the Promising Anti-Mycobacterial Thioridazine
- 2014. Reduced Emergence of Isoniazid Resistance with Concurrent Use of Thioridazine against Acute Murine Tuberculosis