
Inaugural entrants showcase successful new approaches
Thank you to all who submitted entries to the first InnovateTB contest. We are delighted to be able to help promote them as an ongoing effort of the InnovateTB Library.
At the request of our stakeholders, we divided the winners into three categories: Overall Winner, Advocacy Winner (both selected from the popular vote getters by the expert panel), and Fan Favorite (most popular vote getter). We are proud to announce the 2012 InTB Award winners as follows:
Overall Winner |
Advocacy Winner |
Fan Favorite |
|||||||||||

|

|
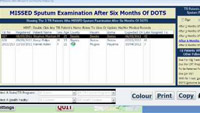
|
|||||||||||
|
Runner Up |
Runner Up |
Runner Up |
|||||||||||
|
|
|
Winners were determined by a combination of popular votes and review by our expert panel members. We would like to thank the panel for their time and guidance:
- Veronique Dartois — Assistant Professor, Public Health Research Institute, UMDNJ
- Lucica Ditiu — Executive Secretary, Stop TB Partnership
- Ken Duncan — Deputy Director, Bill and Melinda Gates Foundation
- Shobha Shukla — Editor and Director Diabetes Media Initiative, Citizen New Service
Beyond the scope of this contest, InTB aims to catalog and promote groundbreaking work in TB diagnosis, control, and treatment. We invite you to continue to submit innovations to our ongoing library — videos, photos or written accounts for TB innovations you have been involved in.
We look forward to seeing more TB Innovations!




