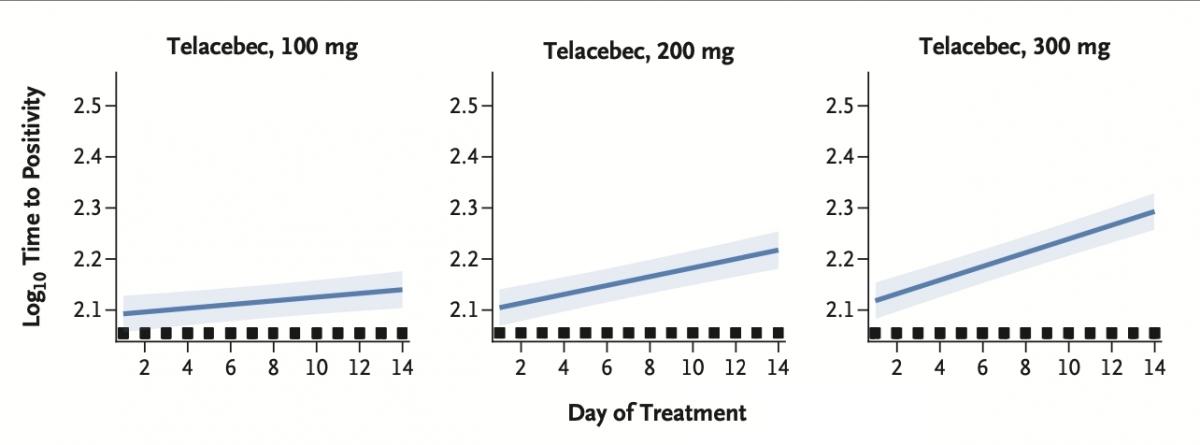
On March 25th, Qurient announced the results of their Phase 2 Clinical Trial (ClinicalTrials.gov number, NCT03563599), which was a prospective, randomized, open-label trial for proof of concept in humans of their novel anti-tuberculosis drug, Telacebec (Q203). The trial involved 61 newly-diagnosed patients with drug-susceptible pulmonary tuberculosis. Patients received a dose of 100 mg, 200 mg, or 300 mg once daily or combination therapy with rifampin, isoniazid, pyrazinamide, and ethambutol (RHZE) as reported in the New England Journal of Medicine. According to the article, increasing doses of telacebec were associated with greater reductions in time to positivity of collected sputum samples, which they used to determine bactericidal activity.
To read more about this latest trial by Qurient, take a look at their press release on Business Wire or their article published in the New England Journal of Medicine.