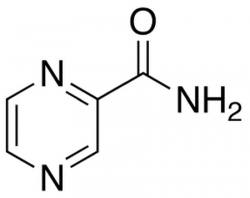
Shi W, Zhang X, Jiang X, Yuan H, Lee JS, Barry CE 3rd, Wang H, Zhang W, Zhang Y. Pyrazinamide Inhibits Trans-Translation in Mycobacterium tuberculosis. Science. 2011 Aug 11. [Epub ahead of print]
This week we highlight an article published out of Ying Zhang’s laboratory at Johns Hopkins University that puts forth a new model for the mode of action of pyrazinamide (PZA) and as well as suggest trans-translation as a potential target to consider in the development of new drugs for TB.
Abstract:
Pyrazinamide (PZA) is a first-line tuberculosis drug that plays a unique role in shortening the duration of tuberculosis chemotherapy. PZA is hydrolyzed intracellularly to pyrazinoic acid (POA) by pyrazinamidase (PZase, encoded by pncA), an enzyme frequently lost in PZA-resistant strains, but the target of POA in Mycobacterium tuberculosis has remained elusive. Here, we identify a new target of POA as the ribosomal protein S1 (RpsA), a vital protein involved in protein translation and the ribosome-sparing process of trans-translation. Three PZA-resistant clinical isolates without pncA mutation harbored RpsA mutations. RpsA overexpression conferred increased PZA resistance, and we confirmed that POA bound to RpsA (but not a clinically identified ΔAla mutant) and subsequently inhibited trans-translation rather than canonical translation. Trans-translation is essential for freeing scarce ribosomes in nonreplicating organisms, and its inhibition may explain the ability of PZA to eradicate persisting organisms.
Note: A patent has been filed by Johns Hopkins University on use of the RpsA as a marker for pyrazinamide resistance and use of the trans-translation pathway as a target for new drugs for TB.
Additional TB R&D News:
Current Opinion in Microbiology: New tuberculosis drugs on the horizon
Bayer HealthCare to provide tuberculosis treatments for China
New Disease Modeling Grants Target Dengue, TB, Other Infections

