 The drug SQ109, developed by Sequella for the treatment of tuberculosis, blocks key biochemical pathways in bacteria, fungi, and parasites. The multiple targets of SQ109 have been determined by researchers at the University of Illinois Urbana-Champaign. The researchers also created analogs of SQ109 that showed potency against a variety of microorganisms. The range of SQ109 activity makes it an attractive broad-base antimicrobial compound. Furthermore, by inhibiting multiple targets, SQ109 reduces the likelihood of resistance developing in bugs. For a link to the paper, click here.
The drug SQ109, developed by Sequella for the treatment of tuberculosis, blocks key biochemical pathways in bacteria, fungi, and parasites. The multiple targets of SQ109 have been determined by researchers at the University of Illinois Urbana-Champaign. The researchers also created analogs of SQ109 that showed potency against a variety of microorganisms. The range of SQ109 activity makes it an attractive broad-base antimicrobial compound. Furthermore, by inhibiting multiple targets, SQ109 reduces the likelihood of resistance developing in bugs. For a link to the paper, click here.
See below for the paper abstract:
Abstract
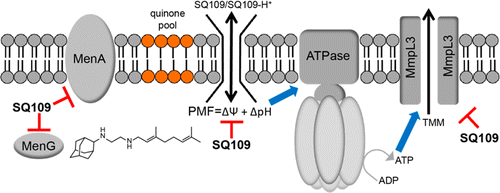
We report the discovery of a series of new drug leads that have potent activity against Mycobacterium tuberculosis as well as against other bacteria, fungi, and a malaria parasite. The compounds are analogues of the new tuberculosis (TB) drug SQ109 (1), which has been reported to act by inhibiting a transporter called MmpL3, involved in cell wall biosynthesis. We show that 1 and the new compounds also target enzymes involved in menaquinone biosynthesis and electron transport, inhibiting respiration and ATP biosynthesis, and are uncouplers, collapsing the pH gradient and membrane potential used to power transporters. The result of such multitarget inhibition is potent inhibition of TB cell growth, as well as very low rates of spontaneous drug resistance. Several targets are absent in humans but are present in other bacteria, as well as in malaria parasites, whose growth is also inhibited.
Additional Coverage and Links
Multitarget TB drug could treat other diseases, evade resistance
TB Drug May Fight Other Diseases Yet Avoid Resistance
Discovery and development of SQ109: a new antitubercular drug with a novel mechanism of action
SQ109 for the Treatment of Tuberculosis
Additional TB R&D News
Benzimidazole-based compounds kill Mycobacterium tuberculosis
The path of anti-tuberculosis drugs: from blood to lesions to mycobacterial cells

