August 14, 2019. FDA Limited Population Approval in combination with bedaquiline and linezolid.
June 6, 2019. FDA Advisory Committee Hearing.
March 8, 2019. TB Alliance Press Release. The TB Alliance’s new drug application (NDA) for the novel tuberculosis (TB) drug candidate pretomanid has been accepted for review by the United States Food and Drug Administration (FDA). The application is for the use of pretomanid as part of a new regimen, in combination with bedaquiline and linezolid, for the treatment of extensively drug-resistant (XDR) TB, treatment intolerant multidrug-resistant (MDR) TB, and treatment non-responsive MDR-TB. The NDA for pretomanid has been granted Priority Review by FDA. The Prescription Drug User Fee Act (PDUFA) action date for an FDA decision is in third quarter 2019.
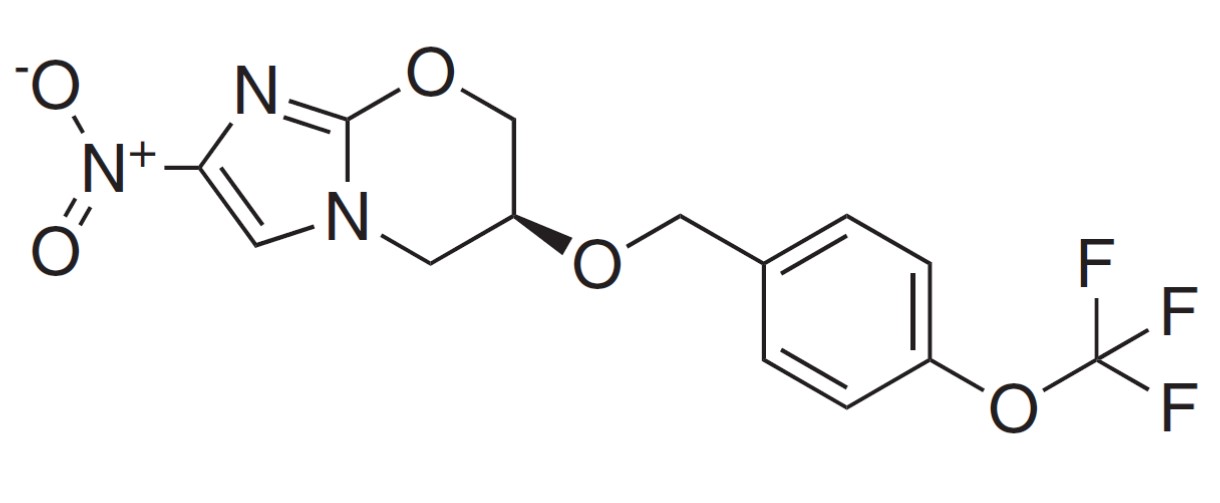
Pretomanid is a nitroimidazole, a class of novel anti-bacterial agents. As a potential TB therapy, it has many attractive characteristics - most notably its novel mechanism of action, its activity in vitro against all tested drug-resistant clinical isolates, and its activity as both a potent bactericidal and a sterilizing agent in mice. In addition, the compound shows no evidence of mutagenicity in a standard battery of genotoxicity studies, no significant cytochrome P450 interactions, and no significant activity against a broad range of Gram-positive and Gram-negative bacteria. This compound has been developed by TB Alliance and is a potential cornerstone of future TB and drug-resistant TB treatment regimens.
| July 5, 2007. Orphan Drug Status granted by FDA. |