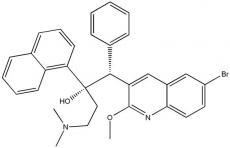
Bedaquiline is a member of the diarylquinoline class of drugs and has a unique mechanism of action, targeting the adenosine triphosphate (ATP) synthase enzyme of the TB mycobacteria. ATP-synthase is used in the process by which M.tb generates its energy supply. It is active against both M.tb and the drug-resistant TB bacteria that cause MDR-TB. Laboratory tests and clinical trials have shown it to have strong bactericidal and sterilizing properties.
In 2012, bedaquiline received conditional approved by the US FDA for the treatment of MDR-TB, in addition to the current second-line treatment regimen. Interim guidance for its use has also been issued by the WHO. Bedaquiline is a potential cornerstone of novel TB regimens currently in development with a novel mechanism of action. As such, could have an even greater impact on TB disease through inclusion in a first-line regimen and/or as a component of a simpler, more affordable drug-resistant TB regimen.